
Mark Nichols
Chair of Biomedical and Health Informatics / Director of Research Development / Associate Professor
School of Medicine
- 816-235-1855
- nicholsmark@umkc.edu
- M5-101
Biography
In 2016, Dr. Nichols joined UMKC as Associate Professor in the Department of Biomedical Sciences at the School of Medicine and as Associate Dean for Research at the School of Nursing and Health Studies. He has been Chair of the Department of Biomedical and Health Informatics since Aug. 2018. He has also been UMKC Interim Vice Chancellor for Research and Economic Development (Jan.-Aug. 2019) to foster collaborative research and grow federal research funding and clinical translational research. He is now Director for Research Development for the UMKC School of Medicine, where he mentors faculty, reviews science and grant applications to federal agencies NIH, NSF, DoD, HRSA. In the past decade, he revised the science/grantsmanship of 96 funded NIH+ applications for > $192 million; many PIs received first-time funding or first renewals.
Med school tenured faculty, department chair, course director, consultant, expert witness, biomedical researcher in molecular biology, pharmacology, oncology, endocrinology for 25 years with focus on estrogens, breast cancer, tamoxifen regulated Cre/FLP DNA recombinases, steroids, protein receptors, gene expression, and precision medicine. He has taught in courses for both M.D. and Ph.D. students each year for two decades, often focusing on basic molecular biology, biochemistry, genetics, endocrinology, and oncology, as well as the pharmacology of estrogens, progestins, contraception, anesthetics, chemotherapy, and precision medicine.
His expertise includes molecular mechanisms of drug and enzyme action, molecular biology, site-directed mutagenesis, gene manipulation and cloning, signal transduction, genomic regulation, cell cycle, steroid hormones, pharmaceutical drug mechanisms, therapeutic development, healthcare, precision medicine oncology; mitochondria, TCA-Krebs cycle, electron transport chain ETC, bioenergetics, and anti-aging; peer-reviewed publications in 20 biomedical journals; inventor on an siRNA biotechnology patent, and research funding from NIH (NIDDK, NCI), DOD (CDRMP), and the American Cancer Society.
Consulting for law firms-biomedical topics-scientific review, search, discovery. Work-product led to win in a lung cancer case; Consultant on estrogen/progestin HRT in breast cancer patients (2 cases); Expert witness in letrozole patent case/generic drug application to FDA with excellent settlement; Consultant on contraceptive hormone risks with third generation progestins.
Honors/Awards
- Grant Reviewer of the Year, 2016
- Pitt Innovator Award (licensed IP to industry, & US Patent 7,524,653), University of Pittsburgh, 2006
- Hillman Fellow in Innovative Cancer Research, University of Pittsburgh Cancer Institute, 2004-2007
Continuing Education
2001- Mini-MBA for Biomedical Scientists (5 months)
- Joseph M. Katz Graduate School of Business, University of Pittsburgh
- From Basic Research to Therapeutic Use: What Every Scientist Needs to Know (3 months)
- Limbach Entrepreneurial Center, University of Pittsburgh Cancer Institute
- Clinical Research Coordinator Training, CTSA (30 hours)
- University of Pittsburgh, School of Medicine, and the Office of Clinical Research
- The Commercialization Pathway: Short course series in Intellectual Property (4 weeks)
- Offices of Enterprise Development, and Technology Management, University of Pittsburgh
- NIH Regional Seminar for NIH Program Funding and Grants, Scottsdale, AZ (3 days)
- NIH Regional Seminar for NIH Program Funding and Grants, Baltimore, MD (3 days)
- Forty NIH and DHHS faculty shared expertise on the NIH strategic goals, new formats, review process, and properties of successful applications to obtain NIH research funds.
- "Grant Writers Seminar and Workshop: Training in the Art of Grantsmanship" presented by GWSW, LLC. Two 8-hour days covering best practices for NIH, NSF, and AHA applications
- Cerner Health Facts Bootcamp for understanding and researching massive EHR databases, UMKC
- Academic Leadership Conference, University of Missouri System, Columbia, MO, August 2019
- Certification for Online Teaching, University of Missouri System, Office of eLearning (6 weeks)
- NIH Regional Seminar for NIH Program Funding and Grants, Bethesda, MD (online 40 hours)
- MEDB 5514 Human Genome Epidemiology (15 weeks)
- UMKC Leadership Development Program (6 sessions, 40 hours)
- Genomic Medicine Short Course, CMKC Genomic Medicine Center (26 hours)
Service
- Interviewer for prospective BA/MD medical students, MMI format (2025->)
- Precision Medicine Tumor Board Review, St. Luke’s System, weekly (2024->)
- Lectures for Faculty Development Seminar program, “How to Construct Competitive Grant Applications for Research Funding" focused on NIH and federal (2012->)
- Facilitated organization of new UMKC School of Science & Engineering (2021-23)
- Member of campus-wide Research Advisory Council (RAC) (2016-23)
- Mentor for Yale PhD graduate and Yale College undergraduate students (2015->)
- Mentor faculty within SOM Faculty Development Program (2018->)
- Member of UMKC Information Services (IS) Advisory Council (2019->)
- Member of Biomedical & Health Informatics admissions Committee for MS and PhD (2019->)
- Research Advisory Council for the UMKC Health Sciences District (12 institutions) (2018->)
- Member of the UMKC Non-Physician Promotion Committee (2016-19)
- Member of the UMKC School of Medicine Committee on PA, AA student progression (2020->)
- UMKC Conflict of Interest Oversight Committee (2019->)
- UMKC Cross-Campus Entrepreneurship Advisory Board, Regnier Institute for Entrepreneurship & Innovation, Bloch School of Management (2017->)
- Course Director for "Clinical Bioinformatics" for MS, PhD, MD students (2022->)
- Course Director for "Responsible Conduct of Research" for MS, PhD, MD students (2020->)
- Steering committee for BioNexus (Kansas City Area Life Sciences) annual conference (CRISPR/cas gene editing 2017; Cancer immunotherapies 2018; Zoonotic diseases 2019) (2017-19)
- Reviewer for UMKC Funding for Excellence grants, SOM Sarah Morrison grants, etc. (2016->)
Selected Publications
Nichols, M. (2015) New directions for drug-resistant breast cancer: the CDK4/6 inhibitors. Future Med Chem. 2015 Aug 7:1473-1481.
Kim SW, Fishilevich E, Arango-Argoty G, Lin Y, Liu G, Li Z, Monaghan AP, Nichols M, & John B. (2015) Genome-wide transcript profiling reveals novel breast cancer-associated intronic sense RNAs. PLoS ONE 10(3): e0120296. doi:10.1371/journal.pone.0120296, March 23, 2015.
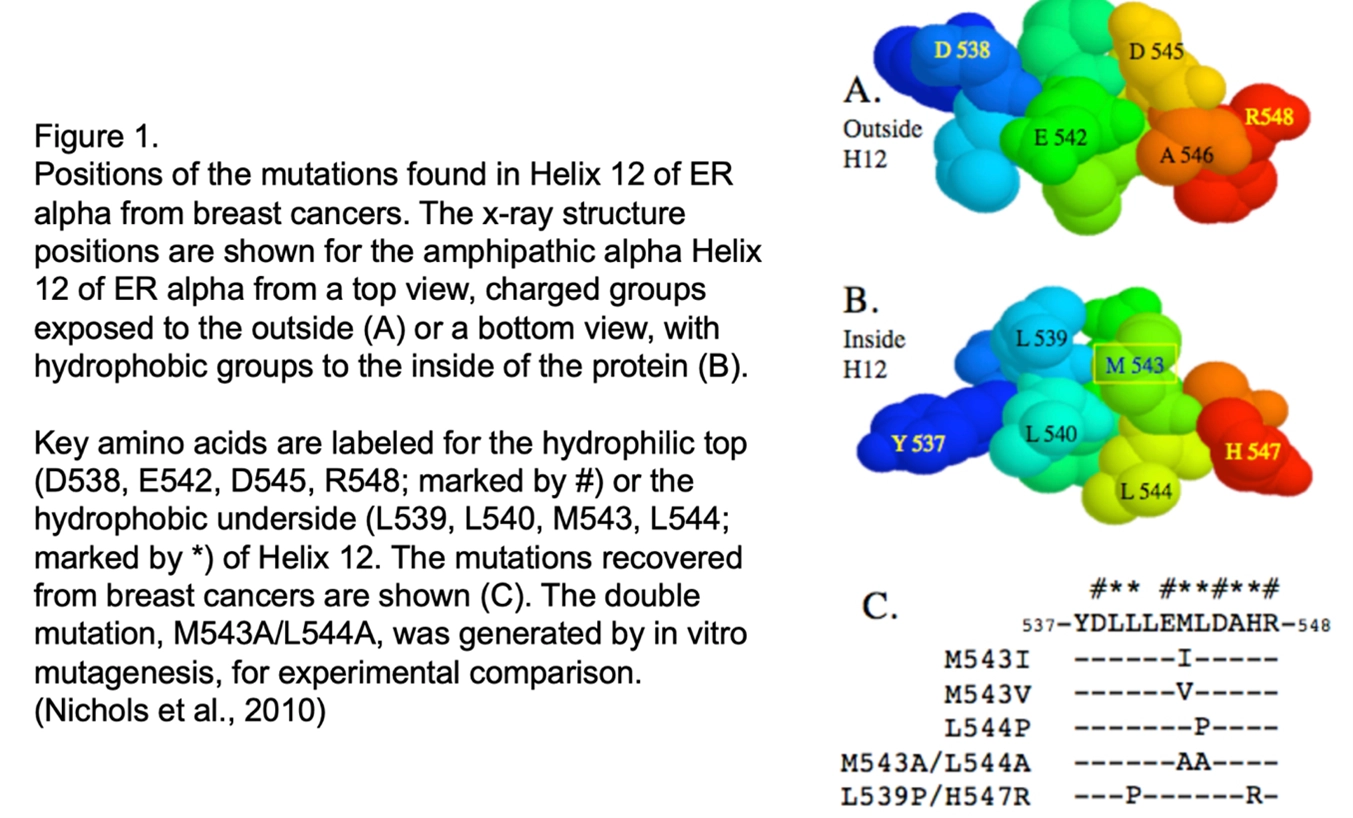
Nichols M, Cheng P, Liu Y, Kanterewicz B, Hershberger PA, McCarty Jr KS. (2010) Breast cancer derived M543V mutation at helix 12 of ERa inverts response to estrogen and SERMs, Breast Cancer Research & Treatment, 120:761-8. [Epub 2009 Jun 13 ahead of print]
Kim SW, Li Z, Moore PS, Monaghan AP, Chang Y, Nichols M, and John B. (2010) A sensitive nonradioactive northern blot method to detect small RNAs. Nucleic Acids Research, 38(7): e98. Epub 2010 Jan 15 (A top ten paper in Nucleic Acids Research, 2010-2012)
Nichols M and Steinman RA. (2009) A recombinase-based palindrome generator capable of producing random shRNA libraries, J Biotechnol, 143:79-84.
Nichols M and Steinman RA. U.S. Patent 7,524,653 (awarded March 2009, filed May 2003). Small interfering RNA libraries and methods of synthesis and use. USPTO, 2009.
Hershberger PA, Stabile LP, Kanterewicz B, Rothstein ME, Gubish CT, Land S, Shuai Y, Siegfried JM, & Nichols M. (2009) Estrogen receptor beta (ERß) subtype-specific ligands increase transcription, p44/p42 mitogen activated protein kinase (MAPK) activation and growth in human non-small cell lung cancer cells. J Steroid Biochem Molec Biol.116: 102-109.
Nichols M. (2007) The fight against tamoxifen resistance in breast cancer therapy: a new target in the battle? Molecular Interventions, 7:13-6.
McCarty Jr KS, Nichols M, Hershberger PA, and McCarty Sr KS. (2006) Principles of Endocrine Therapy, “Progestins”, in Cancer Medicine, 7th edit, Ed. Holland et al., BC Decker Inc., Hamilton, Ontario, Canada, Chapter 55, 843-849
Hershberger PA, Vasquez AC, Kanterewicz B, Land S, Siegfried J, and Nichols M. (2005) Regulation of endogenous gene expression in human non-small cell lung cancer cells by estrogen receptor ligands. Cancer Research, 65: 1598-1605.
Nichols M and McCarty KS Jr. (2002) Functional Mutations of Estrogen Receptor Protein: Assay for detection. Breast Cancer Research & Treatment, 72: 61-68. (Eleven years before novel RNAseq confirmed this model).
Research in Dr. Nichols’ lab involved study of steroid hormone receptors, primarily the estrogen receptors alpha and beta (ERa, ERß), and their role in normal, as well as in cancer tissue. Dr. Nichols developed in vitro model systems that allow (a) the analysis of the effect of estrogen receptor (ER) mutations, some found in breast cancers, on antihormone/SERM resistance and (b) screening of novel compounds for ER-subtype selective ligands. Better understanding of ligand activation of ERs should lead to improved endocrine therapies for treating and perhaps preventing breast and other estrogen responsive cancers.
Links
Education and Training
- B.A., Biochemistry, University of California, Berkeley
- Ph.D., Molecular Biophysics and Biochemistry, Yale University
- Post-doc, German Cancer Research Center (DKFZ), Heidelberg, Germany
- Research Assistant, European Molecular Biology Lab (EMBL), Heidelberg, Germany
- Assistant Professor, Pharmacology and Chemical Biology, University of Pittsburgh, School of Medicine